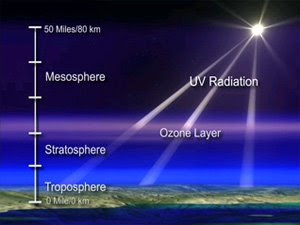

The ozone layer is a layer in Earth's atmosphere which contains relatively high concentrations of ozone (O3). This layer absorbs 93-99% of the sun's high frequency ultraviolet light, which is potentially damaging to life on earth.[1] Over 91% of ozone in earth's atmosphere is present here.[1] "Relatively high" means a few parts per million—much higher than the concentrations in the lower atmosphere but still small compared to the main components of the atmosphere. It is mainly located in the lower portion of the stratosphere from approximately 10 km to 50 km above Earth's surface, though the thickness varies seasonally and geographically.[2] The ozone layer was discovered in 1913 by the French physicists Charles Fabry and Henri Buisson. Its properties were explored in detail by the British meteorologist G. M. B. Dobson, who developed a simple spectrophotometer that could be used to measure stratospheric ozone from the ground. Between 1928 and 1958 Dobson established a worldwide network of ozone monitoring stations which continues to operate today(2008). The "Dobson unit", a convenient measure of the total amount of ozone in a column overhead, is named in his honor.
Origin of ozone
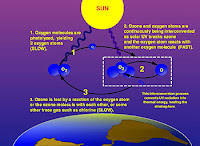
Ozone-oxygen cycle in the ozone layer.
The photochemical mechanisms that give rise to the ozone layer were worked out by the British physicist Sidney Chapman in 1930. Ozone in the earth's stratosphere is created by ultraviolet light striking oxygen molecules containing two oxygen atoms (O2), splitting them into individual oxygen atoms (atomic oxygen); the atomic oxygen then combines with unbroken O2 to create ozone, O3. The ozone molecule is also unstable (although, in the stratosphere, long-lived) and when ultraviolet light hits ozone it splits into a molecule of O2 and an atom of atomic oxygen, a continuing process called the ozone-oxygen cycle, thus creating an ozone layer in the stratosphere, the region from about 10 to 50 km (32,000 to 164,000 feet) above Earth's surface. About 90% of the ozone in our atmosphere is contained in the stratosphere. Ozone concentrations are greatest between about 20 and 40 km, where they range from about 2 to 8 parts per million. If all of the ozone were compressed to the pressure of the air at sea level, it would be only a few millimeters thick.
Ten percent of the ozone in the atmosphere is contained in the troposphere, the lowest part of our atmosphere where all of our weather takes place. Tropospheric ozone has two sources: about 10 % is transported down from the stratosphere while the remainder is created in smaller amounts through different mechanisms.
Ozone-oxygen cycle in the ozone layer.
The photochemical mechanisms that give rise to the ozone layer were worked out by the British physicist Sidney Chapman in 1930. Ozone in the earth's stratosphere is created by ultraviolet light striking oxygen molecules containing two oxygen atoms (O2), splitting them into individual oxygen atoms (atomic oxygen); the atomic oxygen then combines with unbroken O2 to create ozone, O3. The ozone molecule is also unstable (although, in the stratosphere, long-lived) and when ultraviolet light hits ozone it splits into a molecule of O2 and an atom of atomic oxygen, a continuing process called the ozone-oxygen cycle, thus creating an ozone layer in the stratosphere, the region from about 10 to 50 km (32,000 to 164,000 feet) above Earth's surface. About 90% of the ozone in our atmosphere is contained in the stratosphere. Ozone concentrations are greatest between about 20 and 40 km, where they range from about 2 to 8 parts per million. If all of the ozone were compressed to the pressure of the air at sea level, it would be only a few millimeters thick.
Ten percent of the ozone in the atmosphere is contained in the troposphere, the lowest part of our atmosphere where all of our weather takes place. Tropospheric ozone has two sources: about 10 % is transported down from the stratosphere while the remainder is created in smaller amounts through different mechanisms.
Origin of Ozone
Ultraviolet light and ozone
Levels of ozone at various altitudes and blocking of ultraviolet radiation.
Although the concentration of the ozone in the ozone layer is very small, it is vitally important to life because it absorbs biologically harmful ultraviolet (UV) radiation emitted from the Sun. UV radiation is divided into three categories, based on its wavelength; these are referred to as UV-A, UV-B, and UV-C. UV-C, which would be very harmful to humans, is entirely screened out by ozone at around 35 km altitude. UV-B radiation can be harmful to the skin and is the main cause of sunburn; excessive exposure can also cause genetic damage, as a result problems such as skin cancer. The ozone layer is very effective at screening out UV-B; for radiation with a wavelength of 290 nm, the intensity at Earth's surface is 350 billion times weaker than at the top of the atmosphere. Nevertheless, some UV-B reaches the surface. Most UV-A reaches the surface; this radiation is significantly less harmful, although it can potentially cause genetic damage.
Depletion of the ozone layer allows more of the UV radiation, and particularly the more harmful wavelengths, to reach the surface, causing increased genetic damage to living creatures and organisms.
Levels of ozone at various altitudes and blocking of ultraviolet radiation.
Although the concentration of the ozone in the ozone layer is very small, it is vitally important to life because it absorbs biologically harmful ultraviolet (UV) radiation emitted from the Sun. UV radiation is divided into three categories, based on its wavelength; these are referred to as UV-A, UV-B, and UV-C. UV-C, which would be very harmful to humans, is entirely screened out by ozone at around 35 km altitude. UV-B radiation can be harmful to the skin and is the main cause of sunburn; excessive exposure can also cause genetic damage, as a result problems such as skin cancer. The ozone layer is very effective at screening out UV-B; for radiation with a wavelength of 290 nm, the intensity at Earth's surface is 350 billion times weaker than at the top of the atmosphere. Nevertheless, some UV-B reaches the surface. Most UV-A reaches the surface; this radiation is significantly less harmful, although it can potentially cause genetic damage.
Depletion of the ozone layer allows more of the UV radiation, and particularly the more harmful wavelengths, to reach the surface, causing increased genetic damage to living creatures and organisms.
DNA sensitivity to UV
UV energy levels at several altitudes. Blue line shows DNA sensitivity. Red line shows surface energy level with 10% decrease in ozone.
To appreciate the importance of this ultraviolet radiation screening, we can consider a characteristic of radiation damage called an action spectrum. An action spectrum gives us a measure of the relative effectiveness of radiation in generating a certain biological response over a range of wavelengths. This response might be erythema (sunburn), changes in plant growth, or changes in molecular DNA. Certain wavelengths of UV radiation have a much greater probability of DNA damage than others. Fortunately, where DNA is easily damaged, such as by wavelengths shorter than 290 nm, ozone strongly absorbs UV. At the longer wavelengths where ozone absorbs weakly, DNA damage is less likely.
UV energy levels at several altitudes. Blue line shows DNA sensitivity. Red line shows surface energy level with 10% decrease in ozone.
To appreciate the importance of this ultraviolet radiation screening, we can consider a characteristic of radiation damage called an action spectrum. An action spectrum gives us a measure of the relative effectiveness of radiation in generating a certain biological response over a range of wavelengths. This response might be erythema (sunburn), changes in plant growth, or changes in molecular DNA. Certain wavelengths of UV radiation have a much greater probability of DNA damage than others. Fortunately, where DNA is easily damaged, such as by wavelengths shorter than 290 nm, ozone strongly absorbs UV. At the longer wavelengths where ozone absorbs weakly, DNA damage is less likely.
Distribution of ozone in the stratosphere
Global monthly average total ozone amount
The thickness of the ozone layer—that is, the total amount of ozone in a column overhead—varies by a large factor worldwide, being in general smaller near the equator and larger as one moves towards the poles. It also varies with season, being in general thicker during the spring and thinner during the autumn. The reasons for this latitude and seasonal dependence are complicated, involving atmospheric circulation patterns as well as solar intensity.
Since stratospheric ozone is produced by solar UV radiation, one might expect to find the highest ozone levels over the tropics and the lowest over polar regions. The same argument would lead one to expect the highest ozone levels in the summer and the lowest in the winter. The observed behavior is very different: most of the ozone is found in the mid-to-high latitudes of the northern and southern hemispheres, and the highest levels are found in the spring, not summer, and the lowest in the autumn, not winter. During winter, the ozone layer actually increases in depth. This puzzle is explained by the prevailing stratospheric wind patterns, known as the Brewer-Dobson circulation. While most of the ozone is indeed created over the tropics, the stratospheric circulation then transports it poleward and downward to the lower stratosphere of the high latitudes.
Brewer-Dobson circulation in the ozone layer.
The ozone layer is higher in altitude in the tropics, and lower in altitude in the extratropics, especially in the polar regions. This altitude variation of ozone results from the slow circulation that lifts the ozone-poor air out of the troposphere into the stratosphere. As this air slowly rises in the tropics, ozone is produced by the overhead sun which photolyzes oxygen molecules. As this slow circulation bends towards the mid-latitudes, it carries the ozone-rich air from the tropical middle stratosphere to the mid-and-high latitudes lower stratosphere. The high ozone concentrations at high latitudes are due to the accumulation of ozone at lower altitudes.
The Brewer-Dobson circulation moves very slowly. The time needed to lift an air parcel from the tropical tropopause near 16 km (50,000 ft) to 20 km is about 4-5 months (about 30 feet (9.1 m) per day). Even though ozone in the lower tropical stratosphere is produced at a very slow rate, the lifting circulation is so slow that ozone can build up to relatively high levels by the time it reaches 26 km.
Ozone amounts over the continental United States (25°N to 49°N) are highest in the northern spring (April and May). These ozone amounts fall over the course of the summer to their lowest amounts in October, and then rise again over the course of the winter. Again, wind transport of ozone is principally responsible for the seasonal evolution of these higher latitude ozone patterns.
The total column amount of ozone generally increases as we move from the tropics to higher latitudes in both hemispheres. However, the overall column amounts are greater in the northern hemisphere high latitudes than in the southern hemisphere high latitudes. In addition, while the highest amounts of column ozone over the Arctic occur in the northern spring (March-April), the opposite is true over the Antarctic, where the lowest amounts of column ozone occur in the southern spring (September-October). Indeed, the highest amounts of column ozone anywhere in the world are found over the Arctic region during the northern spring period of March and April. The amounts then decrease over the course of the northern summer. Meanwhile, the lowest amounts of column ozone anywhere in the world are found over the Antarctic in the southern spring period of September and October, owing to the ozone hole phenomenon.
Global monthly average total ozone amount
The thickness of the ozone layer—that is, the total amount of ozone in a column overhead—varies by a large factor worldwide, being in general smaller near the equator and larger as one moves towards the poles. It also varies with season, being in general thicker during the spring and thinner during the autumn. The reasons for this latitude and seasonal dependence are complicated, involving atmospheric circulation patterns as well as solar intensity.
Since stratospheric ozone is produced by solar UV radiation, one might expect to find the highest ozone levels over the tropics and the lowest over polar regions. The same argument would lead one to expect the highest ozone levels in the summer and the lowest in the winter. The observed behavior is very different: most of the ozone is found in the mid-to-high latitudes of the northern and southern hemispheres, and the highest levels are found in the spring, not summer, and the lowest in the autumn, not winter. During winter, the ozone layer actually increases in depth. This puzzle is explained by the prevailing stratospheric wind patterns, known as the Brewer-Dobson circulation. While most of the ozone is indeed created over the tropics, the stratospheric circulation then transports it poleward and downward to the lower stratosphere of the high latitudes.
Brewer-Dobson circulation in the ozone layer.
The ozone layer is higher in altitude in the tropics, and lower in altitude in the extratropics, especially in the polar regions. This altitude variation of ozone results from the slow circulation that lifts the ozone-poor air out of the troposphere into the stratosphere. As this air slowly rises in the tropics, ozone is produced by the overhead sun which photolyzes oxygen molecules. As this slow circulation bends towards the mid-latitudes, it carries the ozone-rich air from the tropical middle stratosphere to the mid-and-high latitudes lower stratosphere. The high ozone concentrations at high latitudes are due to the accumulation of ozone at lower altitudes.
The Brewer-Dobson circulation moves very slowly. The time needed to lift an air parcel from the tropical tropopause near 16 km (50,000 ft) to 20 km is about 4-5 months (about 30 feet (9.1 m) per day). Even though ozone in the lower tropical stratosphere is produced at a very slow rate, the lifting circulation is so slow that ozone can build up to relatively high levels by the time it reaches 26 km.
Ozone amounts over the continental United States (25°N to 49°N) are highest in the northern spring (April and May). These ozone amounts fall over the course of the summer to their lowest amounts in October, and then rise again over the course of the winter. Again, wind transport of ozone is principally responsible for the seasonal evolution of these higher latitude ozone patterns.
The total column amount of ozone generally increases as we move from the tropics to higher latitudes in both hemispheres. However, the overall column amounts are greater in the northern hemisphere high latitudes than in the southern hemisphere high latitudes. In addition, while the highest amounts of column ozone over the Arctic occur in the northern spring (March-April), the opposite is true over the Antarctic, where the lowest amounts of column ozone occur in the southern spring (September-October). Indeed, the highest amounts of column ozone anywhere in the world are found over the Arctic region during the northern spring period of March and April. The amounts then decrease over the course of the northern summer. Meanwhile, the lowest amounts of column ozone anywhere in the world are found over the Antarctic in the southern spring period of September and October, owing to the ozone hole phenomenon.
Source : wikipedia and pictures from yahoo!